- Pages are currently viewing
- Home > Undergraduate Schools > List of Medicine > Department of Biochemistry
Department of Biochemistry
Outline
In 1945, Professor Yashiro Kotake, the first President of the school at that time, started this department. Since then, Professors Yahito Kotake, Kazuo Hotta, Katashi Ichihara, Yuichi Matsumura, Ryo Kido, and Morimitsu Nishikimi successively served as the chairman of the department, and Professor Yoshito Ihara has been the chairman since 2007.
Educational Outline
We teach courses in the following educational programs.
- Biochemistry and Metabolism (for 2nd grade students of the School of Medicine)
- Molecular Cellular Biology (for 2nd grade students of the School of Medicine)
- Metabolism in the Cell (for students of the Graduate School of Medicine)
Research Outline
Our work is focused on clarifying the molecular mechanisms involved in the post-translational regulation of glycoproteins in cells, and their roles in cell biology, physiology and pathology. We have several main research topics, which concern [1] C-mannosyl tryptophan, a unique post-translational modification in cellular proteins, and [2] calreticulin, a lectin chaperone regulating quality control of glycoprotein biosynthesis and Ca2+ homeostasis in the endoplasmic reticulum. The third research topic is to elucidate molecular mechanisms of human diseases from the view point of protein aggregation ([3]). We are also working on how aberrant lipid droplet accumulation affect cancer pathologies ([4]).
[1] Protein C-mannosylation is unique in that an a・mannose attaches directly to the indole C2 carbon atom of a tryptophan residue through a C-C bond. C-Mannosylation usually occurs at the first tryptophan in the consensus amino acid sequence Trp-x-x-Trp (W-x-x-W) through an enzymatic reaction with a specific mannosyltransferase, which has yet to be identified. Most substrates for C-mannosylation are part of either the thrombospondin type-1 repeat (TSR) superfamily or cytokine receptor family, suggesting a functional role for C-mannosylation in specific substrates. Site-directed mutagenesis in the W-x-x-W motif has revealed C-mannosylation to be important in the folding or targeting of substrate proteins, such as mucins and ADAMTS-like 1, in the cell. Furthermore, we identified Hsc70 as a protein specifically bound to C-mannosylated TSR-derived peptides, and found that the interaction of Hsc70 with C-mannosylated peptides enhanced the TNF-a・producing signaling by Hsc70 in macrophage-like cells. These findings suggest that the C-mannosylation of some target proteins plays pivotal functional roles in the cell. We examine the role of C-mannosylation in proteins using molecular biological, biochemical, immunological and biophysical techniques.
Our research goal is to clarify the molecular mechanism of protein C-mannosylation and its physiological or pathological functions in the cell.
Selected References
- Tabata S, Yamashita Y, Inai Y, Morita S, Kosako H, Takagi T, Shide K, Manabe S, Matsuoka TA, Shimoda K, Sonoki T, Ihara Y, Tamura S. C-Mannosyl tryptophan is a novel biomarker for thrombocytosis of myeloproliferative neoplasms. Sci Rep., 14, 18858, 2024.
- Nishitsuji K, Ikezaki M, Manabe S, Uchimura K, Ito Y, Ihara Y. Thrombospondin type 1 repeat-derived C-mannosylated peptide attenuates synaptogenesis of cortical neurons induced by primary astrocytes via TGF-beta. Glycoconj J., 39, 701-710, 2022.
- Ikezaki M, Nishitsuji K, Matsumura K, Manabe S, Shibukawa Y, Wada Y, Ito Y, Ihara Y. C-Mannosylated tryptophan-containing WSPW peptide binds to actinin-4 and alters E-cadherin subcellular localization in lung epithelial-like A549 cells. Biochimie., 192, 136-146, 2022.
- Minakata S, Manabe S, Inai Y, Ikezaki M, Nishitsuji K, Ito Y, Ihara Y. Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine. Molecules., 26, 5258, 2021.
- Morita S, Inai Y, Minakata S, Kishimoto S, Manabe S, Iwahashi N, Ino K, Ito Y, Akamizu T, Ihara Y. Quantification of serum C-mannosyl tryptophan by novel assay to evaluate renal function and vascular complications in patients with type 2 diabetes. Sci Rep., 11, 1946, 2021.
- Minakata S, Inai Y, Manabe S, Nishitsuji K, Ito Y, Ihara Y, Monomeric C-mannosyl tryptophan is a degradation product of autophagy in cultured cells. Glycoconj J. 37, 635-645, 2020.
- Inai Y, Ueda K, Matsui IL, Tajiri M, Minakata S, Wada Y, Ihara Y, Role of C-mannosylation in the secretion of mindin. Biochim Biophys Acta Gen Subj., 1864, 129632, 2020.
- Iwahashi N, Inai Y, Minakata S, Sakurai S, Manabe S, Ito Y, Ino K, Ihara Y, C-Mannosyl tryptophan increases in the plasma of patients with ovarian cancer. Oncol Lett., 19, 908-916, 2020.
- Sakurai S, Inai Y, Minakata S, Manabe S, Ito Y, Ihara Y, A novel assay for detection and quantification of C-mannosyl tryptophan in normal or diabetic mice. Sci Rep., 9, 4675, 2019.
- Ihara Y, Inai Y, Ikezaki M, Matsui I.-L, Manabe S, and Ito Y, C-Mannosylation: a modification on tryptophan in cellular proteins. In “Glycoscience: Biology and Medicine” (Eds.) Taniguchi N., Endo T., Hart G.W., Seeberger P., and Wong C.-H., Springer-Verlag GmbH, Heidelberg, Germany, 1091-1099, 2015.
- Protein C-mannosylation and its prospective functions in the cell.: Ihara Y., Inai Y., and Ikezaki M. Trends Glycosci. Glycotechnol., 23, 1-13, 2011.
- C-Mannosylated peptides derived from the thrombospondin type 1 repeat interact with Hsc70 to modulate its signaling in RAW264.7 cells.: Ihara Y., Manabe S., Ikezaki M., Inai Y., Matsui I.-S. L., Ohta Y., Muroi E., and Ito Y. Glycobiology, 20, 1298-1310, 2010.
- C-Mannosylated peptides derived from the thrombospondin type 1 repeat enhance lipopolysaccharide-induced signaling in macrophage-like RAW264.7 cells.: Muroi E., Manabe S., Ikezaki M., Urata Y., Sato S., Kondo T., Ito Y., and Ihara Y. Glycobiology, 17, 1015-1028, 2007.
[2] Calreticulin is a Ca2+-binding multifunctional molecular chaperone in the endoplasmic reticulum. Calreticulin is involved in a variety of cellular processes including the quality control of glycoprotein synthesis in the endoplasmic reticulum, Ca2+ homeostasis, intracellular signaling, gene expression, and nuclear transport. In cancer cells, the altered expression of calreticulin may lead to alterations in cellular characteristics, such as growth, adhesion, motility, immune responses, and susceptibility to apoptosis.
Previously, we reported that overexpression of calreticulin through gene transfection caused epithelial-mesenchymal transition (EMT)-like changes in epithelia-derived Madin-Darby Canine Kidney cells. The EMT is a crucial process controlling the morphogenesis of multicellular organisms during embryogenesis, development, and disease states such as malignancies, chronic inflammation, and tissue fibrosis. In the study, we found that overexpression of calreticulin repressed E-cadherin gene expression through up-regulation of its repressor, Slug, via altered Ca2+ homeostasis. These results suggested a novel function of calreticulin related to EMT-like changes of cellular phenotype. We examine the role of calreticulin in the EMT machinery using molecular biological, biochemical, immunological and biophysical techniques.
Our research goal is to clarify the unrevealed mechanism of calreticulin-induced EMT and its physiological or pathological relevance to cellular functions.
Selected References
- Matsukawa H, Ikezaki M, Nishioka K, Iwahashi N, Fujimoto M, Nishitsuji K, Ihara Y, Ino K. Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells. Biomolecules., 12, 1050, 2022.
- Iwahashi N, Ikezaki M, Nishitsuji K, Yamamoto M, Matsuzaki I, Kato N, Takaoka N, Taniguchi M, Murata SI, Ino K, Ihara Y. Extracellularly Released Calreticulin Induced by Endoplasmic Reticulum Stress Impairs Syncytialization of Cytotrophoblast Model BeWo Cells. Cells., 10, 1305, 2021.
- Ihara Y, Ikezaki M, Takatani M, Ito Y, Calnexin/Calreticulin and Assays Related to N-Glycoprotein Folding In Vitro. Methods Mol Biol., 2132, 295-308, 2020.
- Ikezaki M, Minakata S, Nishitsuji K, Tabata S, Lee Matsui IS, Takatani M, Usukura J, Ito Y, Ihara Y, Calreticulin protects insulin against reductive stress in vitro and in MIN6 cells. Biochimie., 171-172, 1-11, 2020.
- Iwahashi N, Ikezaki M, Matsuzaki I, Yamamoto M, Toujima S, Murata SI, Ihara Y, Ino K, Calreticulin regulates syncytialization through control of the synthesis and transportation of E-cadherin in BeWo cells. Endocrinology., 160, 359-374, 2019.
- Yamamoto M, Ikezaki M, Toujima S, Iwahashi N, Mizoguchi M, Nanjo S, Minami S, Ihara Y, Ino K, Calreticulin Is Involved in Invasion of Human Extravillous Trophoblasts Through Functional Regulation of Integrinβ1. Endocrinology., 158, 3874-3889, 2017.
- Epithelial calreticulin up-regulation promotes profibrotic responses and tubulointerstitial fibrosis development.: Prakoura N., Politis P.K., Ihara Y., Michalak M., and Charonis A.S. Am. J. Pathol., 183, 1474-1487, 2013.
- Alteration of integrin-dependent adhesion and signaling in EMT-like MDCK cells established through overexpression of calreticulin.: Ihara Y., Inai Y., and Ikezaki M. J. Cell. Biochem., 112, 2518-2528, 2011.
- Calreticulin represses E-cadherin gene expression in MDCK cells via slug.: Hayashida Y., Urata Y., Muroi E., Kono T., Miyata Y., Nomata K., Kanetake H., Kondo T., and Ihara Y. J. Biol. Chem., 281, 32469-32484, 2006.
- Calreticulin, a molecular chaperone in the endoplasmic reticulum, modulates radiosensitivity of human glioblastoma U251MG cells.: Okunaga T., Urata Y., Goto S., Matsuo T., Mizota S., Tsutsumi K., Nagata I., Kondo T., and Ihara Y. Cancer Res., 66, 8662-8671, 2006.
[3] Proteins usually exist in properly folded native states. When a protein fails to maintain its native conformation, the protein begins to aggregate. Protein aggregates are sometimes even more stable than are proteins in the native state. Therefore, protein misfolding and protein aggregation cause a broad range of diseases called protein misfolding diseases or conformational diseases. Conformational diseases are conditions that are associated with deposition of protein aggregates in or around organs such as the liver, spleen, and brain. More than 50 human diseases are thought to be associated with deposition of protein aggregates. One of our main research interests is the possible transmission of amyloid diseases by a prion-like mechanism. Previous studies have suggested the presence of unidentified in vivo cofactors of amyloid deposition and propagation in addition to proteins that form amyloid. By collaborating with Univ. Lille, CNRS, UMR 8576-UGSF (Unité de Glycobiologie Structurale et Fonctionnelle), we are putting particular focus on sulfated glycosaminoglycans (GAGs), which are common non-protein components of amyloid deposits in systemic and localized amyloidoses, as the possible common mediator of the propagation of protein aggregates. Another our main purpose is to investigate the pathology of human disease, that have not been considered as conformational diseases, from the viewpoint of protein aggregation. For example, we are investigating the pathology of p53-mutated cancers. We found that cytoplasmic accumulation of mutant p53 aggregates is associated with poor prognosis in several cancers, and are working on the pathological roles of p53 aggregates in these cancers.
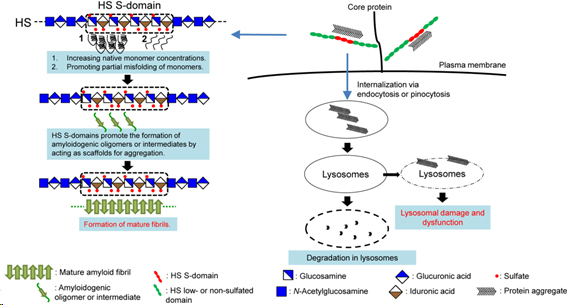
Nishitsuji et al., Trends in Glycoscience and Glycotechnology, Vol. 33 No. 196 E1–E5 (2021)
Possible roles of sulfated GAGs in the pathology of amyloidoses. Sulfated GAGs, especially highly sulfated domains of heparan sulfate act as scaffolds for aggregation (left). Sulfated GAGs also can act as receptors for protein aggregates (right).
Selected References
- Nishitsuji K, Mito R, Ikezaki M, Yano H, Fujiwara Y, Matsubara E, Nishikawa T, Ihara Y, Uchimura K, Iwahashi N, Sakagami T, Suzuki M, Komohara Y. Impacts of cytoplasmic p53 aggregates on the prognosis and the transcriptome in lung squamous cell carcinoma. Cancer Sci., 115, 2947-2960, 2024.
- Iwahashi N, Ikezaki M, Komohara Y, Fujiwara Y, Noguchi T, Nishioka K, Sakai K, Nishio K, Ueda M, Ihara Y, Uchimura K, Ino K, Nishitsuji K. Cytoplasmic p53 aggregates accumulated in p53-mutated cancer correlate with poor prognosis. PNAS Nexus., 1, pgac128, 2022.
- Nishitsuji K, Uchimura K: Contribution of Sulfated Glycosaminoglycans to the Pathology of Amyloidosis. Trends in Glycoscience and Glycotechnology 2021, 33:E141-E5.
- Iwahashi N, Ikezaki M, Saito H, Uchimura K, Nishitsuji K: Cell-to-cell transmission of p53 aggregates: a novel player in oncology? Mol Cell Oncol 2021, 8:1892444.
- Iwahashi N, Ikezaki M, Nishikawa T, Namba N, Ohgita T, Saito H, Ihara Y, Shimanouchi T, Ino K, Uchimura K, Nishitsuji K: Sulfated glycosaminoglycans mediate prion-like behavior of p53 aggregates. Proc Natl Acad Sci U S A 2020, 117:33225-34.
- Kameyama H, Uchimura K, Yamashita T, Kuwabara K, Mizuguchi M, Hung SC, Okuhira K, Masuda T, Kosugi T, Ohgita T, Saito H, Ando Y, Nishitsuji K: The Accumulation of Heparan Sulfate S-Domains in Kidney Transthyretin Deposits Accelerates Fibril Formation and Promotes Cytotoxicity. Am J Pathol 2019, 189:308-19.
- Nishitsuji K: Heparan sulfate S-domains and extracellular sulfatases (Sulfs): their possible roles in protein aggregation diseases. Glycoconj J 2018, 35:387-96.
[4] Alterations in lipid metabolism is one of the characteristics of human cancers. Although abnormal lipid accumulation in malignant tumors has been recognized many years ago, its effects and roles in cancer pathology are yet to be elucidated. We are now working in association with Department of Obstetrics and Gynecology at Wakayama Medical University, Department of Diagnostic Pathology at Kyoto University, and Department of Cell Pathology at Kumamoto University, and are seeking novel therapeutic strategies for malignant tumors by targeting aberrant lipid metabolism.
Selected References
- Iwahashi N, Ikezaki M, Fujimoto M, Komohara Y, Fujiwara Y, Yamamoto M, Mizoguchi M, Matsubara K, Watanabe Y, Matsuzaki I, Murata SI, Ihara Y, Ino K, Nishitsuji K: Lipid Droplet Accumulation Independently Predicts Poor Clinical Prognosis in High-Grade Serous Ovarian Carcinoma. Cancers (Basel) 2021, 13:5251.
- Fujimoto M, Matsuzaki I, Nishitsuji K, Yamamoto Y, Murakami D, Yoshikawa T, Fukui A, Mori Y, Nishino M, Takahashi Y, Iwahashi Y, Warigaya K, Kojima F, Jinnin M, Murata SI: Adipophilin expression in cutaneous malignant melanoma is associated with high proliferation and poor clinical prognosis. Lab Invest 2020, 100:727-37.
- Ohmoto T, Nishitsuji K, Yoshitani N, Mizuguchi M, Yanagisawa Y, Saito H, Sakashita N: K604, a specific acylCoA:cholesterol acyltransferase 1 inhibitor, suppresses proliferation of U251MG glioblastoma cells. Molecular medicine reports 2015, 12:6037-42.
Contact
- Yoshito Ihara, Professor
Department of Biochemistry, Wakayama Medical University School of Medicine, - 811-1 Kimiidera, Wakayama 641-8509, Japan.
Phone/Fax: 81-73-441-0628
E-mail: y-ihara(at)wakayama-med.ac.jp

