生体調節機構研究部
教室概要
本教室では、免疫応答、炎症応答を制御する分子機構を解明するための研究を主に遺伝子改変マウスを用いて行っています。
医学部学生教育では、免疫学、分子生物学の基本的な知識、考え方と共に、最先端の知見も紹介し、医学研究者としての基本的な姿勢を学んでもらうことを目標にしています。
場所:和歌山県立医科大学 研究棟2F
研究概要
概要
多細胞生物は、外界からのシグナルを受け取り、シグナル伝達機構、転写因子の活性化を通じて、種々の生物学的機能を発揮します。当研究室では、哺乳類の免疫応答機構がどのようなシグナルで制御されているかを、抗原提示細胞、樹状細胞に焦点を当てて、分子、細胞、個体の種々のレベルで研究を展開しています。
研究内容
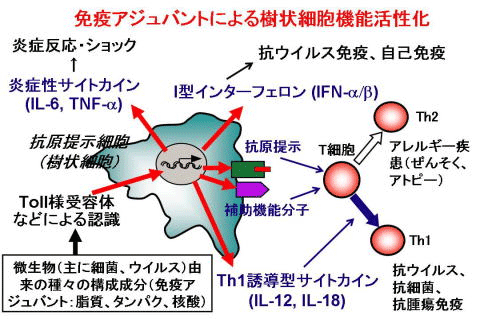
現在、研究室では、哺乳類の生体防御機構、特に、自然免疫と獲得免疫との協調作用を担う、樹状細胞に興味を持って研究を進めています。自然免疫は、ショウジョウバエから哺乳類まで保存されたシステムで、病原体を認識し、種々のサイトカインを産生することにより、病原体の迅速な排除に関与しています。また、T細胞の活性化、分化の方向付けをすることにより、獲得免疫の樹立にも必須の役割を果たしています。樹状細胞は、この一連の過程に必須の抗原提示細胞で、微生物由来の種々の成分(免疫アジュバント)を、Toll様受容体(TLR)を含む、種々の受容体を介して認識することにより機能します。この機構は感染や腫瘍に対する生体防御に必須ですが、バランスが崩れると、アレルギーや自己免疫など種々の病態も引き起こします。さらに、最近、種々の内因性物質、環境物質にも応答し、炎症病態を引き起こすこともわかってきました。この機構を解明することにより、新たな免疫制御手段、炎症制御手段の獲得に結びつく成果が期待されます。我々は、種々の免疫アジュバント、遺伝子改変マウスを用いて、樹状細胞活性化機構に関与する機能分子を明らかにし、免疫応答、炎症応答を制御する手段の獲得を目指しています。
スタッフ紹介(2023年5月現在)
メールアドレスは、後ろに@wakayama-med.ac.jpを付けてください。
| 役職 | 氏名 | メールアドレス |
|---|---|---|
| 教授 | 改正 恒康 | tkaisho |
| 講師 | 佐々木 泉 | izumisas |
| 助教 | 加藤 喬 | ktakashi |
| 事業担当補助職員 | 服部 郁子 | coco6425 |
| 事業担当補助職員 | 岡本 千珠代 | okamotoc |
| 秘書 | 大倉 抄美子 | okura |
研究業績
- K. English, R. Kwan, L. E. Holz, C. McGuffog, J.M.M. Krol, D. Kempe, T. Kaisho, W.R. Heath, L. Lisowski, M. Biro, G.W. McCaughan, D.G. Bowen, P. Bertolino. 2024. A hepatic network of dendritic cells mediates CD4 T cell help outside lymphoid organs. Nat Commun 10;15:1261.
- V. E. Domenjo, V. Casella, R. Iwabuchi, E. Fossum, M. Pedragosa, Q. Castellví, P. R. Cebollada, T. Kaisho, K. Terahara, G. Bocharov, J. Argilaguet, A. Meyerhans. 2022. XCR1+ DCs are critical for T cell-mediated immunotherapy of chronic viral infections. Cell Rep. 14;42:112123.
- R. Kaczmarek, A. R. Piñeros, P. E. Patterson, T. B. Bertolini, G. Q. Perrin, A. Sherman, J. Born, S. Arisa, M. C. Arvin, M. M. Kamocka, M. M. Martinez, K. W. Dunn, S. M. Quinn, J. J. Morris, A. R. Wilhelm, T. Kaisho, M. M. Melero, M. Biswas, M. H. Kaplan, A. K. Linnemann, L. A. George, R. M. Camire, R. W. Herzog. 2023. Factor VIII trafficking to CD4+ T cells shapes its immunogenicity and requires several types of antigen-presenting cells. Blood. 20;142:290-305.
- M. Ugur, R J. Labios, C. Fenton, K. Knöpper, K. Jobin, F. Imdahl, G. Golda, K. Hoh, A. Grafen, T. Kaisho, A. E. Saliba, D. Grün, G. Gasteiger, M. Bajénoff, W. Kastenmüller. 2023. Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks. Immunity. 8;56:1778-1793.e10.
- SRP. Kumar, M. Biswas, D. Cao, S. Arisa, MM. Melero, AK. Lam, AR. Piñeros, R. Kapur, T. Kaisho, RJ. Kaufman, W. Xiao, DM. Shayakhmetov, C. Terhorst, YP. de Jong, RW. Herzog. 2023. TLR9-independent CD8+ T Cell Responses in Hepatic AAV Gene Transfer Through IL-1R1-MyD88 Signaling. Mol Ther. 5:S1525-0016(23)00662-7
- H. Saiga, M. Ueno, T. Tanaka, T. Kaisho, K. Hoshino. 2022. Transcription factor MafB-mediated inhibition of type I interferons in plasmacytoid dendritic cells. Int. Immunol. 34:159-172.
- N. Kanazawa, H. Hemmi, N. Kinjo, H. Ohnishi, J. Hamazaki, H. Mishima, A. Kinoshita, T. Mizushima, S. Hamada, K. Hamada, N. Kawamoto, S. Kadowaki, Y. Honda, K. Izawa, R. Nishikomori, M. Tsumura, Y. Yamashita, S. Tamura, T. Orimo, T. Ozasa, T. Kato, I. Sasaki, Y. Fukuda-Ohta, N. Wakaki-Nishiyama, Y. Inaba, K. Kunimoto, S. Okada, T. Taketani, K. Nakanishi, S. Murata, K. -I. Yoshiura, T. Kaisho. 2021. Heterozygous missense variant of the proteasome subunit β-type 9 causes neonatal-onset autoinflammation and immunodeficiency. Nat Commun. 24;12:6819.
- S. Dähling, A. M. Mansilla, K. Knöpper, A. Grafen, D. T Utzschneider, M. Ugur, P. G Whitney, A. Bachem, P. Arampatzi, F. Imdahl, T. Kaisho, D. Zehn, F. Klauschen, N. Garbi, A. Kallies, A.-E. Saliba, G. Gasteiger, S. Bedoui, W. Kastenmüller. 2022. Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity 55:656-670.e8.
- A. L. Burrack, Z. C. Schmiechen, M. T. Patterson, E. A. Miller, E. J. Spartz, M. R. Rollins, J. F. Raynor, J. S. Mitchell, T. Kaisho, B. T. Fife, I. M. Stromnes. 2022. Distinct myeloid antigen-presenting cells dictate differential fates of tumor-specific CD8+ T cells in pancreatic cancer. JCI Insight. 8;7:e151593.
- K. English, S. Y. Tan, R. Kwan, L. E. Holz, F. Sierro, C. McGuffog, T. Kaisho, W. R. Heath, K. P. MacDonald, G. W. McCaughan, D. G. Bowen, P. Bertolino. 2022. The liver contains distinct interconnected networks of CX3CR1+ macrophages, XCR1+ type 1 and CD301a+ type 2 conventional dendritic cells embedded within portal tracts. Immunol Cell Biol. 100:394-408
- S. Badrinath, M. O. Dellacherie, A. Li, S. Zheng, X. Zhang, M. Sobral, J. W. Pyrdol, K. L. Smith, Y. Lu, S. Haag, H. Ijaz, F. Connor-Stroud, T. Kaisho, G. Dranoff, G. C. Yuan, D. J. Mooney, K. W. Wucherpfennig. 2022. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature. 606:992-998.
- D. A Michelson, K. Hase, T. Kaisho, C. Benoist, D. Mathis. 2022. Thymic epithelial cells co-opt lineage-defining transcription factors to eliminate autoreactive T cells. Cell. 7;185:2542-2558.e18.
- A. Teijeira, S. Garasa, C. luri, C. de Andrea, M. Gato, C. Molina, T. Kaisho, A. Cirella, A. Azpilikueta, S. Wculek, J. Egea, I. Olivera, I. Rodriguez, A. Rouzaut, V. Verkhusha, K. Valencia, D. Sancho, P. Berraondo, I. Melero. 2022. Depletion of cDC1 cells upon immunotherapy of established tumors precludes efficacy. Cancer Res. 2;82:4373-4385.
- N. Ishihara, Y. Nakamura, K. Yakabe, S. Komiyama, Y. Fujimura, T. Kaisho, S. Kimura, K. Hase. 2022. Spi-B alleviates food allergy by securing mucosal barrier and immune tolerance in the intestine. Front Allergy. 6;3:996657.
- T. Tanioku, M. Nishibata, Y. Tokinaga, K. Konno, M. Watanabe, H. Hemmi, Y. Fukuda-Ohta, T. Kaisho, H. Furue, T. Kawamata. 2022. Tmem45b is essential for inflammation- and tissue injury-induced mechanical pain hypersensitivity. Proc Natl Acad Sci U S A. 8;119:e2121989119.
- M. C. Alcantara, K. Suzuki, A. R. Acebedo, D. Kajioka, S. Hirohata, T. Kaisho, Y. Hatano, K. Yamagata, S. Takahashi, G. Yamada. 2022. Androgen-regulated MafB drives cell migration via MMP11-dependent extracellular matrix remodeling in mice. iScience. 16;25:105609.
- T. Moriya, K. Kitagawa, Y. Hayakawa, H. Hemmi, T. Kaisho, S. Ueha, R. Ikebuchi, I. Yasuda, Y. Nakanishi, T. Honda, K. Matsushima, K. Kabashima, M. Ueda, Y. Kusumoto, T. Chtanova, M. Tomura. 2021. Immunogenic tumor cell death promotes dendritic cell migration and inhibits tumor growth via enhanced T cell immunity. iScience. 14;24:102424
- T. B. Bertolini, J. L. Shirley, I. Zolotukhin, X. Li, T. Kaisho, W. Xiao, S. Kumar, R. W. Herzog. 2021. Effect of CpG Depletion of Vector Genome on CD8 + T Cell Responses in AAV Gene Therapy. Front Immunol. 31;12:672449.
- T. Kato, M. Yamamoto, T. Orimo, I. Sasaki, K. Murakami, H. Hemmi, Y. Fukuda-Ohta, K. Isono, S. Takayama, H. Nakamura, Y. Otsuki, T. Miyamato, J. Takita, T. Yasumi, R. Nishikomori, T. Matsubayashi, K. Izawa, T. Kaisho. 2021. Augmentation of STING-induced type I interferon production in COPA syndrome. Arthritis Rheumatol. 73:2105-2115.
- Á de Mingo Pulido, K. Hanggi, A. Gardner, J. Li, D. P. Celias, B. B. Bittencourt, E. Mohamed, J. T. Tinoco, O. Osunmakinde, R. Peña, T. Kaisho, J. Kaufmann, K. McEachern, H. Soliman, V. C. Luca, P. C. Rodriguez, X. Yu, B. Ruffell. 2021. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity. 8;54:1154-1167.e7.
- S. Stutte, J. Ruf, I. Kugler, H. Ishikawa-Ankerhold, A. Blutke, P. Marconi, T. Maeda, T. Kaisho, A. Krug, B. Popper, H. Lauterbach, M. Colonna, U. von Andrian, T. Brocker. 2021. Type I interferon mediated induction of somatostatin leads to suppression of ghrelin and appetite thereby promoting viral immunity in mice. Brain Behav Immun. 95:429-443.
- D. Hashimoto, J. G. R. Colet, A. Murashima, K. Fujimoto, Y. Ueda, K. Suzuki, T. Hyuga, H. Hemmi, T. Kaisho, S. Takahashi, Y. Takahama, G. Yamada. 2021. Radiation inducible MafB gene is required for thymic regeneration. Sci Rep. 17;11:10439
- K. M. Audsley, T. Wagner, C. Ta, H. V. Newnes, A. C. Buzzai, S. A. Barnes, B. Wylie, J. Armitage, T. Kaisho, A. Bosco, A. McDonnell, M. Cruickshank, V. S. Fear, B. Foley, J. Waithman. 2021. IFNβ Is a Potent Adjuvant for Cancer Vaccination Strategies. Front Immunol. 6;12:735133.
- S. Kimura, Y. Nakamura, N. Kobayashi, K. Shiroguchi, E. Kawakami, M. Mutoh, H. Iwanaga, T. Yamada, M. Hisamoto, M. Nakamura, N. Udagawa, S. Sato, T. Kaisho, T. Iwanaga, K. Hase. 2020. Osteoprotegerin-dependent M-cell self-regulation balances gut infection and immunity. Nat Commun. 11:234.
- Y. Nakamura, H. Mimura, J. Kunisawa, Y. Furusawa, D. Takahashi, Y. Fujimura, T. Kaisho, H. Kiyono, K. Hase. 2020. Microfold Cell-Dependent Antigen Transport Alleviates Infectious Colitis by Inducing Antigen-Specific Cellular Immunity. Mucosal Immunol. 13:679-690.
- Y. Mizumoto, H. Hemmi, M. Katsuda, M. Miyazawa, Y. Kitahata, A. Miyamoto, M. Nakamori, T. Ojima, K. Matsuda, M. Nakamura, K. Hayata, Y. Fukuda-Ohta, M. Sugiyama, T. Ohta, T. Orimo, S. Okura, I. Sasaki, K. Tamada, H. Yamaue, T. Kaisho. 2020. Anticancer effects of chemokine-directed antigen delivery to a cross-presenting dendritic cell subset with immune checkpoint blockade. Br J Cancer 122:1185-1193.
- R. Miyazaki, H. Saiga, T. Kato, T. Bakoshi, R. Senba, A. Shintani, M. Suzuki, K. Takao, I. Sasaki, A. Iizuka, M. Sugiyama, N. Iwami, Y. Fukuda-Ohta, H. Hemmi, T. Tanaka, M. Miyake, T. Kaisho, K. Hoshino. 2020. The mechanism of action of Spi-B in the transcriptional activation of the interferon-α4 gene. Biochem Biophys Res Commun. 525:477-482.
- D. Ashour, P. Arampatzi, V. Pavlovic, K. U Förstner, T. Kaisho, A. Beilhack, F. Erhard, M. B Lutz. 2020. IL-12 From Endogenous cDC1, and Not Vaccine DC, Is Required for Th1 Induction, JCI Insight 5:135143.
- Y. Kato, T. M. Steiner, H. Park, R. O. Hitchcock, A. Zaid, J. Liang Hor, S. Devi, G. M. Davey, D. Vremec, K. M. Tullett, P. S. Tan, F. Ahmet, S. N. Mueller, S. Alonso, D. M. Tarlinton, H. L. Ploegh, T. Kaisho, L. Beattie, J. H. Manton, D. Fernandez-Ruiz, K. Shortman, M. H. Lahoud, W. R. Heath and I. Caminschi. 2020. Display of native antigen on cDC1 that have spatial access to both T and B cells underlies efficient humoral vaccination. J Immunol 205:1842-1856.
- A. Jodo, A. Shibazaki, A. Onuma, T. Kaisho, and T. Tanaka. 2020. PDLIM7 synergizes with PDLIM2 and p62/Sqstm1 to inhibit inflammatory signaling by promoting degradation of the p65 Subunit of NF-κB. Front Immunol. 11:1559.
- T. Tanoue, S. Morita, D. R. Plichta, A. N. Skelly, W. Suda, Y. Sugiura, S. Narushima, H. Vlamakis, I.Motoo, K. Sugita, A. Shiota, K. Takeshita, K. Yasuma, D. Riethmacher, T. Kaisho, J. M. Norman, D. Mucida, M. Suematsu, T. Yaguchi, V. Bucci, T. Inoue, Y. Kawakami, B. Olle, B. Roberts, M. Hattori, R. J. Xavier, K. Atarashi, K. Honda. 2019. A defined commensal consortium induces CD8 T cells and anti-cancer immunity. Nature 565:600-605.
- T. Orimo, I. Sasaki, H. Hemmi, T. Ozasa, Y. Fukuda-Ohta, T. Ohta, M. Morinaka, M. Kitauchi, T. Yamaguchi, Y. Sato, T. Tanaka, K. Hoshino, K. I. Katayama, S. Fukuda, K. Miyake, M. Yamamoto, T. Satoh, K. Furukawa, E. Kuroda, K. J. Ishii, K. Takeda, T. Kaisho. 2019. Cholera toxin B induces interleukine-1 production from resident peritoneal macrophages through pyrin as well as NLRP3 inflammasome. Int Immunol. 31:657-668. (Selected as 2019 International Immunology Outstanding Merit Award)
- K. Ikumi, M. Odanaka, H. Shime, M. Imai, S. Osaga, O. Taguchi, E. Nishida, H. Hemmi, T. Kaisho, A. Morita, S. Yamazaki. 2019. Hyperglycemia is associated with psoriatic inflammation in both humans and mice. J Invest Dermatol 139:1329-1338.e7.
- S. Kimura, N. Kobayashi, Y. Nakamura, T. Kanaya, D. Takahashi, R. Fujiki, M. Mutoh, Y. Obata, T. Iwanaga, T. Nakagawa, N. Kato, S. Sato, T. Kaisho, H. Ohno, K. Hase. 2019. Sox8 is essential for M-cell maturation to accelerate IgA response at the early stage after weaning in mice. J Exp Med 216:831-846.
- Y. Yamashita, A. Nishikawa, Y. Iwahashi, M. Fujimoto, I. Sasaki, H. Mishima, A. Kinoshita, H. Hemmi, N. Kanazawa, K. Ohshima, K.‑I. Imadome. S.‑i. Murata, K.‑i. Yoshiura, T. Kaisho, T. Sonoki, S. Tamura. 2019. Identification of a novel CCDC22 mutation in a patient with severe Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis and aggressive natural killer cell leukemia. Int J Hematol 109:744-750.
- T. Yahata, M. Mizoguchi, A. Kimura, T. Orimo, S. Toujima, Y. Kuninaka, M. Nosaka, Y. Ishida, I. Sasaki, Y. Fukuda-Ohta, H. Hemmi, N. Iwahashi, T. Noguchi, T. Kaisho, T. Kondo, K. Ino. 2019.
- Argilaguet. J, Pedragosa. M, Esteve-Codina. A, Riera. G, Vidal. E, Peligero-Cruz. C, Casella. V, Andreu. D, Kaisho. T, Bocharov. G, Ludewig. B, Heath. S, Meyerhans. A. 2019. Systems analysis reveals complex biological processes during virus infection fate decisions. Genome Res. 29:907-919.
- N. Tsuchiya, R. Zhang, T. Iwama, N. Ueda, T. Liu, M. Tatsumi, Y. Sasaki, R. Shimoda, Y. Osako, Y. Sawada, Y. Kubo, A. Miyashita, S. Fukushima, Z. Cheng, R. Nakaki, K. Takubo, S. Okada, S. Kaneko, H. Ihn, T. Kaisho, Y. Nishimura, S. Senju, I. Endo, T. Nakatsura, Y. Uemura. 2019. Type I interferon delivery by iPSC-derived myeloid cells elicits antitumor immunity via XCR1+ dendritic cells. Cell Rep. 29:162-175.
- S. Yamazaki, M. Odanaka, A. Nishioka, S. Kasuya, H. Shime, H. Hemmi, M. Imai, D. Riethmacher, T. Kaisho, N. Ohkura, S. Sakaguchi and A. Morita. 2018. Ultraviolet B–induced maturation of CD11b-Type langerin− dendritic cells controls the expansion of Foxp3+ regulatory T cells in the skin. J Immunol. 200:119-129.
- Smita Gopinath, Myoungjoo Kim, Tasfia Rakib, Patrick Wong, Michael Van Zandt, Natasha Barry, Tsuneyasu Kaisho, Andrew Goodman, and Akiko Iwasaki. 2018. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat Microbiol 3(5):611-621.
- H. Kayama, M. Kohyama, D. Okuzaki, D. Motooka, S. Barman, R. Okumura, M. Muneta, K. Hoshino, I. Sasaki, W. Ise, H. Matsuno, J. Nishimura, T. Kurosaki, S. Nakamura, H. Arase, T. Kaisho, K. Takeda. 2018. Heme ameliorates dextran sodium sulfate-induced colitis through providing intestinal macrophages with noninflammatory profiles. Proc. Natl. Acad. Sci. USA. 115:8418-8423.
- A. Brewitz, S. Eickhoff, S. Dähling, T. Quast, S. Bedoui, R. A. Kroczek, C. Kurts, N. Garbi, W. Barchet, M. Iannacone, F. Klauschen, W. Kolanus, T. Kaisho, M. Colonna, R. N. Germain, W. Kastenmüller. 2017. CD8+ T cells orchestrate pDC-XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity 46:205-219.
- C. Shimokawa, T. Kanaya, M. Hachisuka, K. Ishiwata, H. Hisaeda, Y. Kurashima, H. Kiyono, T. Yoshimoto, T. Kaisho, H. Ohno. 2017. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity 46:863–874
- D. Fernandez-Ruiz, L. S. Lau, N. Ghazanfari, C. M. Jones, W. Y. Ng, G. M. Davey, D. Berthold, L. Holz2, Y. Kato, M. H. Enders, G. Bayarsaikhan, S. H. Hendriks, L. I. M. Lansink, J. A. Engel, M. S. F. Soon, K. R. James, A. Cozijnsen, V. Mollard, A. D. Uboldi, C. J. Tonkin, T. F. de Koning-Ward, P. R. Gilson, T. Kaisho, A. Haque, B. S. Crabb, F. R. Carbone, G. I. McFadden, W. R. Heath. 2017. Development of a novel CD4+ TCR transgenic line that reveals a dominant role for CD8+ DC and CD40-Signaling in the generation of helper and CTL responses to blood stage malaria. J. Immunol. 199:4165-4179.
- Y. Ishida, A. Kimura, M. Nosaka, Y. Kuninaka, H. Hemmi, I. Sasaki, T. Kaisho, N. Mukaida, T. Kondo. 2017. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration. Sci Rep 7:16833.
- M. Kitano, C. Yamazaki, A. Takumi, T. Ikeno, H. Hemmi, N. Takahashi, K. Shimizu, Scott E. Fraser, K. Hoshino, T. Kaisho, T. Okada. 20165. Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A, early online.
- T. Ohta, M. Sugiyama, H. Hemmi, C. Yamazaki, S. Okura, I. Sasaki, Y. Fukuda, T. Orimo, K. J. Ishii, K. Hoshino, F. Ginhoux, T. Kaisho. 2016. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci. Rep. Mar 23;6:23505.
- E. W. Roberts, M. L. Broz, M. Binnewies, M. B. Headley, A. E. Nelson, D. M. Wolf, T. Kaisho, D. Bogunovic, N. Bhardwaj, M. F. Krummel. 2016. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30:324-336.
- Y. Sato, A. Mii, Y. Hamazaki, H. Fujita, H. Nakata, K. Masuda, S. Nishiyama, S. Shibuya, H. Haga, O. Ogawa, A. Shimizu, S. Narumiya, T. Kaisho, M. Arita, M. Yanagisawa, M. Miyasaka, K. Sharma, N. Minato, H. Kawamoto, M. Yanagita. 2016. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 1:e87680.
- A. Brewitz, S. Eickhoff, S. Dähling, T. Quast, S. Bedoui, R. A. Kroczek, C. Kurts, N. Garbi, W. Barchet, M. Iannacone, F. Klauschen, W. Kolanus, T. Kaisho, M. Colonna, R. N. Germain, W. Kastenmüller. CD8+ T Cells Orchestrate pDC-XCR1+ Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity 46:205-219.
- H. Sasaki, D. Kurotaki, N. Osato, H. Sato, I. Sasaki, S. Koizumi, H. Wang, C. Kaneda, A. Nishiyama, T. Kaisho, H. Aburatani, H. C. Morse III, K. Ozato, T. Tamura. 2015. Transcription factor IRF8 plays a critical role in the development of murine basophils and mast cells. Blood 125:358-369.
- N. J. Daniels, E. Hyde, S. Ghosh, K. Seo, K. M. Price, K. Hoshino, T. Kaisho, T. Okada, F. Ronchese. 2015. Antigen-specific cytotoxic T lymphocytes target airway CD103+ and CD11b+ dendritic cells to suppress allergic inflammation. Mucosal Immunol, early online.
- S. Eickhoff, A. Brewitz, M. Y. Gerner, F. Klauschen, K. Komander1, H. Hemmi, N. Garbi, T. Kaisho, R. N. Germain, W. Kastenmüller. 2015. Robust anti-viral immunity requires multiple distinct t cell-dendritic cell interactions. Cell 162:1322-1337.
- R. Ono, T. Kaisho, T. Tanaka. 2015. PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm. Sci. Rep. 5:18327.
- H. Zhao, T. Aoshi, S. Kawai, Y. Mori, A. Konishi, M. Ozkan, Y. Fujita, Y. Haseda, M. Shimizu, M. Kohyama, K. Kobiyama, K. Eto, J. Nabekura, T. Horii, T. Ishino, M. Yuda, H. Hemmi, T. Kaisho, S. Akira, M. Kinoshita, K. Tohyama, Y. Yoshioka, K. J. Ishii, C. Coban. 2014. Olfactory plays a key role in spatiotemporal pathogenesis of cerebral malaria. Cell Host Microbe 15:551-563.
- T. Liu, Y. Yamaguchi, Y. Shirasaki, K. Shikada, M. Yamagishi, K. Hoshino, T. Kaisho, K. Takemoto, T. Suzuki, E. Kuranaga, O. Ohara, M. Miura. 2014. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Reports 8:974-982.
- Y. Sato, H. Hara, T. Okuno, N. Ozaki, S. Suzuki, T. Yokomizo, T. Kaisho, H. Yoshida. 2014. IL-27 affects helper T cell responses via regulation of PGE2 production by macrophages. Biochem. Biophys. Res. Commun. 451:215-221.
- S. Yamazaki, A. Nishioka, S. Kasuya, N. Ohkura, H. Hemmi, T. Kaisho, O. Taguchi, S. Sakaguchi, A. Morita. 2014. Homeostasis of thymus-derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J Immunol. 193:5488-97.
- N. Akiyama, M. Shinzawa, M. Miyauchi, H. Yanai, R. Tateishi, Y. Shimo, D. Ohshima, K. Matsuo, I. Sasaki, K.Hoshino, G. Wu, S. Yagi, J. Inoue, T. Kaisho, T. Akiyama. 2014. Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. J. Exp. Med. 211:2425-2438.
- T. Tanaka, A. Shibazaki, R. Ono, T. Kaisho. 2014. HSP70 mediates degradation of the p65 subunit of nuclear factor κB to inhibit inflammatory signaling. Sci. Signal. 7(356), ra119.
- A. Okuma, K. Hoshino, T. Ohba, S. Fukushi, S. Aiba, S. Akira, M. Ono, T. Kaisho, T. Muta. 2013. Enhanced Apoptosis by Disruption of the STAT3-IκB-ζ Signaling Pathway in Epithelial Cells Induces Sjögren’s Syndrome-like Autoimmune Disease. Immunity 38:450-460.
- T. Kawashima, A. Kosaka, H. Yan, G. Zijin, R. Uchiyama, R. Fukui, D. Kaneko; Y. Kumagai; D.-J. You, J. Carreras, S. Uematsu, M. H. Jang, O. Takeuchi, T. Kaisho, S. Akira, K. Miyake, H. Tsutsui, T. Saito, I. Nishimura, N. M. Tsuji. 2013. Double-stranded RNA of small intestinal commensal but not pathogenic bacteria triggers TLR3-mediated IFN-β production by dendritic cells. Immunity 38:1187-1197.
- K. Shimizu, M. Asakura, J. Shinga, Y. Sato, S. Kitahara, K. Hoshino, T. Kaisho, S. P. Schonberger, T. Ezaki, S. I. Fujii. 2013. Invariant NKT cells induce plasmacytoid DC cross-talk with conventional DCs for efficient memory CD8+ T cell induction. J. Immunol. 190:5609-5619.
- C. Yamazaki, M. Sugiyama, T. Ohta, H. Hemmi, E. Hamada, I. Sasaki, Y. Fukuda, T. Yano, M. Nobuoka, T. Hirashima, A. Iizuka, K. Sato, T. Tanaka, K. Hoshino, T. Kaisho. 2013. Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J. Immunol. 190:6071-6082.
- H. Hemmi, N. Zaidi, B. Wang, I. Matos, C. Fiorese, A. Lubkin, L. Zbytnuik, K. Suda, K. Zhang, M. Noda, T. Kaisho, R. M. Steinman, J. Idoyaga. 2012. Treml4, an Ig Superfamily Member, Mediates Presentation of Several Antigens to T Cells In Vivo, Including Protective Immunity to HER2 Protein. J. Immunol. 188:1147-1155.
- S. Mizukami, C. Kajiwara, M. Tanaka, T. Kaisho, H. Udono. 2012. Differential MyD88/IRAK4 requirements for cross-priming and tumor rejection induced by heat shock protein 70-model antigen fusion protein. Cancer Sci. 103:851-9.
- T. Kanaya, K. Hase, D. Takahashi, S. Fukuda, K. Hoshino, I. Sasaki, H. Hemmi, K.A. Knoop, N. Kumar, M. Sato, T. Katsuno, O. Yokosuka, K. Toyooka, K. Nakai, A. Sakamoto, Y. Kitahara, T. Jinnohara, S.J. McSorley, T. Kaisho, I.R. Williams, H. Ohno. 2012. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat Immunol. 13:729-36.
- I. Sasaki, K. Hoshino, T. Sugiyama, C. Yamazaki, T. Yano, A. Iizuka, H. Hemmi, T. Tanaka, M. Saito, M. Sugiyama, Y. Fukuda, T. Ohta, K. Sato, A. Ainai, T. Suzuki, H. Hasegawa, N. Toyama-Sorimachi, H. Kohara, T. Nagasawa, T. Kaisho. 2012. Spi-B is critical for plasmacytoid dendritic cell function and development. Blood 120:4733-4743.
- A. Kosaka, H. Yan, S. Ohashi, Y. Gotoh, A. Sato, H. Tsutsui, T. Kaisho, T. Toda, Tsuji NM. 2012. Lactococcus lactis subsp. cremoris FC triggers IFN-γ production from NK and T cells via IL-12 and IL-18. Int Immunopharmacol. 14:729-33.
- T. Tanaka, Y. Yamamoto, R. Muromoto, O. Ikeda, Y. Sekine, M. J. Grusby, T. Kaisho, T. Matsuda. 2011. PDLIM2 inhibits T helper cell development and granulomatous inflammation through degradation of STAT3. Sci. Signal. 4(202), ra85.
- H. Takagi, T. Fukaya, K. Eizumi, Y. Sato, K. Sato, A. Shibazaki, H. Otsuka, A. Hijikata, T. Watanabe, O. Ohara, T. Kaisho, B. Malissen, K. Sato. 2011. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity 35:958-971.
- T. Matsuda, Y. Kido, S.-I. Asahara, T. Kaisho, T. Tanaka, N. Hashimoto, Y. Shigeyama, A. Takeda, T. Inoue, Y. Shibutani, M. Koyanagi, T. Hosooka, M. Matsumoto, H. Inoue, T. Uchida, M. Koike, Y. Uchiyama, S. Akira, M. Kasuga. 2010. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice J. Clin. Invest. 120:115-126.
- K. Gotoh, Y. Tanaka, A. Nishikimi, R. Nakamura, H. Yamada, N. Maeda, T. Ishikawa, K. Hoshino, T. Uruno, Q. Cao, S. Higashi, Y. Kawaguchi, M. Enjoji, R. Takayanagi, T. Kaisho, Y. Yoshikai and Y. Fukui. 2010. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J. Exp. Med. 207:721-730.
- K. Hoshino , I. Sasaki , T. Sugiyama , T. Yano , C. Yamazaki , T. Yasui , H. Kikutani, T. Kaisho. 2010. Cutting edge: Critical role of IkB Kinase a in TLR7/9-Induced type I interferon production by conventional dendritic cells. J. Immunol 184:3341-3345.
- C. Yamazaki, R. Miyamoto, K. Hoshino, Y. Fukuda, I. Sasaki, M. Saito, H. Ishiguchia, T. Yano, T. Sugiyama, H. Hemmi, T. Tanaka, E. Hamada, T. Hirashima, R. Amakawa, S. Fukuharab, S. Nomura, T. Ito, T. Kaisho, 2010. Conservation of a chemokine system, XCR1 and its ligand, XCL1, between human and mice. Biochem. Biophys. Res. Commun. 397:756-761.
- H. Amuro, T. Ito, R. Miyamoto, H. Sugimoto, Y. Torii, Y. Son, N. Nakamichi, C. Yamazaki, K. Hoshino, T. Kaisho, Y. Ozaki, M. Inaba, R. Amakawa, S. Fukuhara. 2010. Statins, inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, function as inhibitors of cellular and molecular components involved in type I interferon production. Arthritis Rheum. 62:2073-2085.
- R. Miyamoto, T. Ito, S. Nomura, R. Amakawa, H. Amuro, Y. Katashiba, M. Ogata, N. Murakami, K. Shimamoto, C. Yamazaki, K. Hoshino, T. Kaisho, S. Fukuhara. 2010. Inhibitor of IkappaB kinase activity, BAY11-7082, interferes with the interferon regulatory factor 7 nuclear translocation and type I IFN production by plasmacytoid dendritic cells. Arthritis Research Therap. 12:R87.
